Tag Archives: Notice
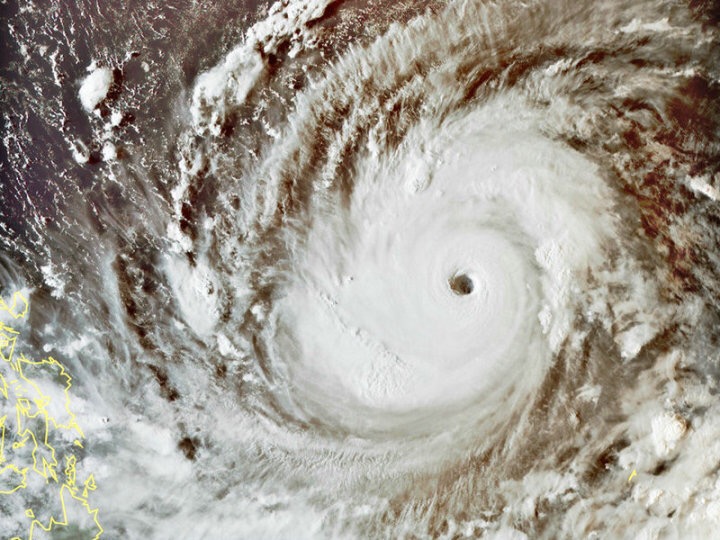
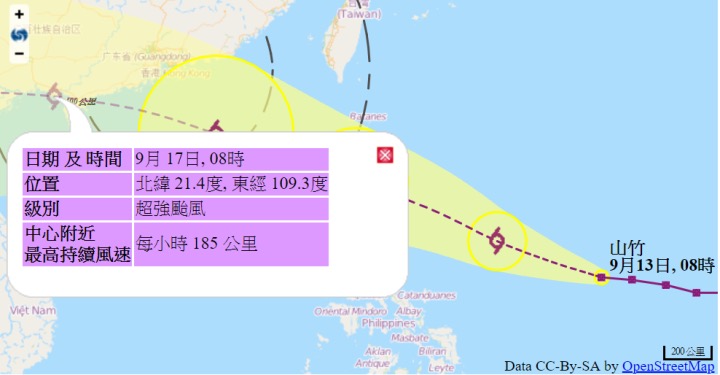
This year's Typhoon"mangosteen"prevention tips
The present O experienced in the previous year Typhoon this year after the current super Typhoon according the Hong Kong Observatory's latest forecast on Macau's influence still exists, although on a days pigeon Typhoon for Macao severely affected, and many pharmaceutical firms and pharmacy personnel to bring the inventory of the drug status of the work and clean shop and work environment.
On the figure for Hong Kong of"mangosteen"route prediction, substantially in the Macau Outer 150km after
Although the latest forecast of the"mangosteen"on the Macau influence is not"day of the Dove"route close to the Australian, but the trade name and the pharmacy personnel should be pre-placed on the ground or near the ground of the drug to move away from, to reduce the burst to a storm surge of drug damage and reduce economic loss, drug professionals should be to the residents to remind the pre-note themselves taking the drug the amount of is sufficient, such as inadequate should be in before the weekend to buy the right amount, in order to avoid suspension during the Typhoon the lack of drug so stop the drug, but also to remind the Don't panic store a large number of drugs in the home, in order to avoid drug store more than valid.
The Macau health Bureau announced the continuing professional development of the test plan
Macau health Bureau in 2015 to the health professionals explain medical professionals when presented with each of the Registered Professionals required for continuing professional development plan(CPD plan), and in the Today's Department of Health to registered the medical professionals sent letters, informing the CPD plan was officially launched.
This plan is experimental, so the current year(by 2018/7-month-2019/6 month)between the CPD requirements and does not affect the renewal of the license, professionals may voluntarily put eligible CPD supporting documents to the Department of Health license section or the professional belongs to the licensing Agency. This year the test may also be so that the agent is the professional community for the new health care professional Act among the CPD requirements are more clearly aware of the process and prepare for the transition to the new Act came into force among the mandatory in-service CPD programme.
The current CPD plan for substantially 1 hour to 1 credit, but because CPD credits included in the project vary, mainly according to the health Bureau files in the content calculation. Each professional per year of CPD credits are different to the pharmacy professional, the pharmacist is temporarily the annual need 25 credits, and a pharmacy technician for 20 credits.
Response Pharmacy Professional higher number and for the local agent personnel professional promotion is the one of the purposes of the future this will also be the opening of more events and conferences, to allow more experts to share their research and clinical treatment experience. That the Macao public to have better drug safety and with the latest scientific research standards.
For CPD details can beClick on the CPD plan page

The US FDA announced the recovery Daclizumab (Zinbryta)
The United States Drug and Food Administration(FDA)in the recently announced full recovery of the United States all of Daclizumab formulation, recovery is the FDA to enforce and assist the drug companies(Biogen And AbbVie)to recall all containing Daclizumab medicines.
Daclizumab trade name Zinbryta is a treatment for relapsing multiple sclerosis Multiple Sclerosis, And Daclizumab is a novel fully human source of anti-Tac monoclonal antibody, and its role in IL-2 receptacle α chain. And in this month 2, there are reports of this drug affects the liver and immune problems. In Europe appeared a total of eight cases of severe side effects, and administration of the factory statement that Daclizumab will be in the global recovery and provide only Daclizumab to 2018 4 June 30 to have the clinical needs of the institution.
And in the Macau market, containing Daclizumab formulation is part of hospital medicine(UH), and the inlet makes for Roche, on the Macau market recovery or not invited to the Macau health Bureau query, while the patient to your attending doctor queries.

Pharmaceutical Affairs notices-doping medicine ingredients
Due to Hong Kong of the Drug Monitoring Department issued a notice,”Allmax Rapidcuts Shredded” within Western medicine ingredient-Yohimbine(Alpha-adrenoreceptor blocker).
But the products and no imports in Macau in batch, such as the citizens there are taking this product, immediately stop taking it, and to pharmaceutical professionals in the query.
Pharmaceutical Affairs notices-drug recovery
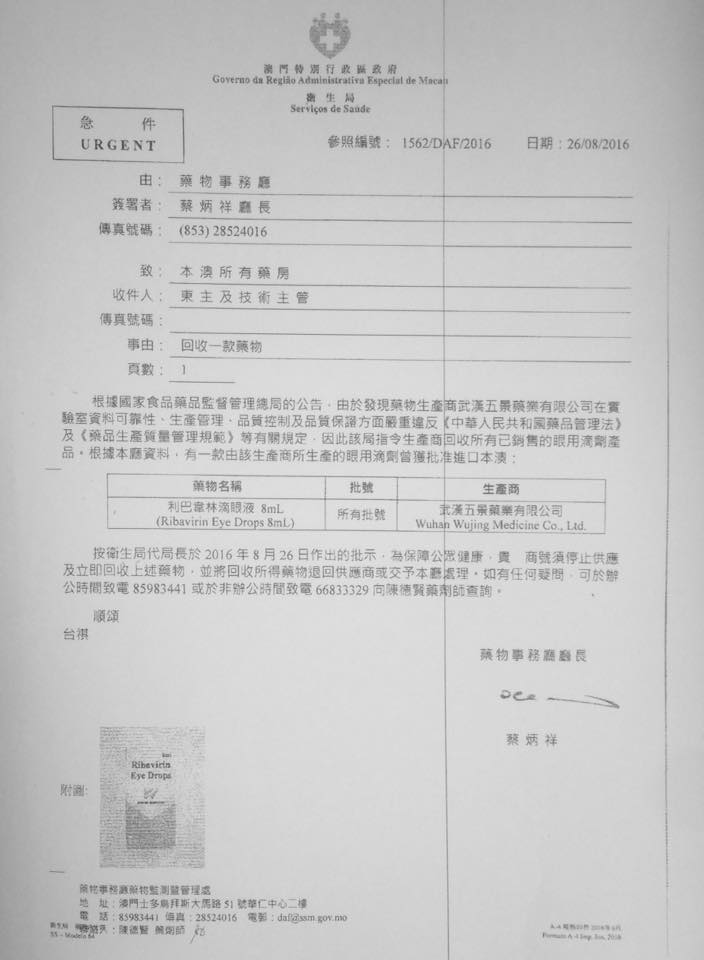
Due to the producers location of the Drug Monitoring Department ordered the producers to recover all production of ophthalmic preparations
Ribavirin eye drops 8ml
Ribavirin Eye Drop 8ml
The US FDA announced drug recovery
American drug recovery matters:
By the USA FDA in 2016 2 December approved the listing of the Cetylev effervescent tablets need to be recovered.
Since the drug packaging is not completely of reason and recycle.
Recovery batch number as follows:
Lot # 005C16, Exp date 2/2018
Lot # 006C16, Exp date 2/2018
Lot # 007C16, Exp date 2/2018
Cetylev(Acetylcysteine) 500mg effervescent tablets the main approved indications for the prevention and reduction of drug Paracetamol (acetaminophen)overdose and cause potential liver damage(hepatotoxic quantity of acetaminophen,APAP)
Acetylcysteine main role is to maintain and restore glutathione(Glutathione)levels, thereby reducing Paracetamol (acetaminophen)is the active metabolite of toxicity.
Pharmaceutical Affairs notices-drug recovery
Pharmaceutical Affairs announcement
Due to Taiwan Food and Drug Administration issued notice,
Producers voluntarily recycle the following products
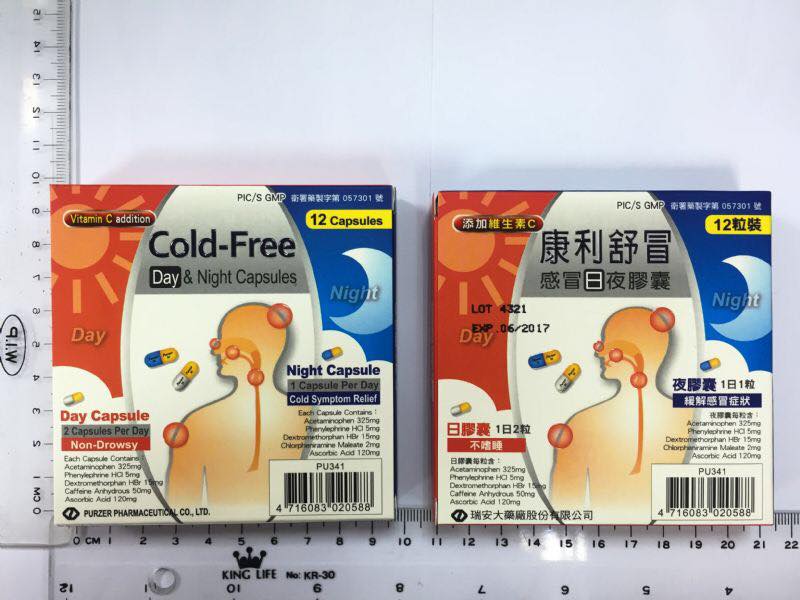
Conley Shu take flu day and night capsules
Cold Free Day n Night Capsules
Manufacturer: Taiwan Ryan large pharmaceutical factory Co., Ltd.
Batch: 4321
Pharmaceutical Affairs notices-drug recovery
Pharmaceutical Affairs announcement
Yongkang pharmaceutical entrance of the merchants-voluntarily recycle the following products
The Major Poly-Vita Drops 50ml
Batch: 20201601
20201603