The Macau administration will complete discussion on the medical malpractice act, related administrative rules and regulations

The administration will complete discussion of“medical malpractice Law”of the three administrative regulations, the health care provider professional liability compulsory insurance act, the medical malpractice test Committee, the medical dispute mediation centre, will be held this month twenty-six days to take effect. The health care provider professional liability compulsory insurance act administrative regulations, healthcare providers must be forced to buy insurance, according to the different classification of the sum insured under limit of fifty thousand Yuan to two million yuan.
And associated administrative rules have been signed completed, pending next Wednesday's Gazette, you can determine the compulsory insurance of the relevant rules.
But the temporary provisions as follows:(by the Executive Council spokesman Liang Qing court in the news will be on offer)
“Recommendations healthcare providers professional liability compulsory insurance coverage including: for medical service providers to the fault of the violation of Health Regulations, guidelines, ethical principles, technical expertise or conventional made to the medical act to the detriment of the clinic's physical or mental health and lead to liability for damages; medical service providers due to the life or physical integrity to serious hazard for the person to provide emergency medical assistance lead to liability for damages; according to the insurance terms of the contract, because the insurance accident compensation and the resulting litigation costs, attorney fees and other expenses. While the set does not belong to insurance coverage of the variety of circumstances, such as due to a medical service provider intentionally act or omission caused the injury, or due to a healthcare provider in a drunken, drug made under the act.”
In addition, the Board instructed the medical records, management, storage and destruction procedures guidelines for medical record requests made to the specification. And Annex VIII for the prescribed contents of the specification, pharmaceutical and medicine who, although in the prescription to write on name and signature.
The following is the Board of Health instructed the content
《Marijuana is not a drug series
In fact, cannabis in medicinal properties on other than a restricted Poison be much higher.
Therefore, the United States, some States are classified as drugs control, but not every pharmacy can be supplied to the patient, so patients need only to specially provide cannabis formulation pharmacy purchase.
Relive the knowledge:
Parkinson's disease(Parkinson’s disease (PD) patients, the largest feature occurs when the EPS(Extrapyramidal Syndromes, or called extrapyramidal symptoms, which factor is the brain neurotransmitter of the proportion of abnormalities in 5-HT increase or Dopamine to reduce in order to make the brain 5-HT and Dopamine relative proportions of change and emergence, but to note the emergence of EPS does not necessarily have Parkinson's.
While cannabis is the main ingredient THC(THC), you can make the neurons to secrete more Dopamine so that the brain transfer of substances the ratio of re-to give balance and order to the EPS symptoms reduced.
The Macao government health Bureau of drug regulations seminar
The Macao government health Bureau of drug regulations briefing。
The following is the registration method, each member should name this briefing close to the wire
Register inference should be pharmaceutical advertising law 30/95/M)within the content
World drug day
World drug day is by FIP(International Pharmaceutical Federation)since 2009 made. Year 9 month 25 day of the conclusion of world drug day. To promote pharmacy to the public.
And present today are attended by the Macau medical community Federation held a seminar to absorb the new drug and disease research knowledge, in the session on acute heart failure of new drugs(Levosimedan)use, etc. topics
Pharmaceutical Affairs announcement-drug safety information
Pharmaceutical Affairs announcement-drug safety information
Due to information drug interactions the recent increase in risk-tips for the pharmacy technician note
Interesting drug share
Pills dissolve believe everyone in the University curriculum experiment has ever seen.
But there is a part of the drug factory for the production of pills or the exterior of the capsule is not dissolved in the body and the discharge, by itself the agent design is to make the users taking daily just a grain of it is sufficient or have other special considerations. So usually labeled as XL, EL, EX, LA, CR, etc., because it is the pharmaceutical factory registration name is different, this is because the commercial marketing considerations, but refers to long-term(Prolonged-release,Extended release etc.)
But not all long-acting(Prolonged-release)drug the outside will not dissolve, mainly the drug companies drug design.
Common:Adalat OROS 30mg(otherwise 60mg, but note that Adalat 5mg does not belong to the long-term, and the drug factory has been discontinued 5mg)
Macau the medical malpractice Act has been Journal Constitution announced
Earlier time, the medical malpractice Act has been gazetted it.
Among the display, all licensed medical personnel are required to purchase mandatory insurance, but all the details of the additional administrative announced
But believe premiums should need 4-digit year.
Link to publicationContent
Pharmaceutical Affairs notices-doping medicine ingredients
Due to Hong Kong of the drug Monitoring Department issued a notice,”Allmax Rapidcuts Shredded” within Western medicine ingredient-Yohimbine(Alpha-adrenoreceptor blocker).
But the products and no imports in Macau in batch, such as the citizens there are taking this product, immediately stop taking it, and to pharmaceutical professionals in the query.
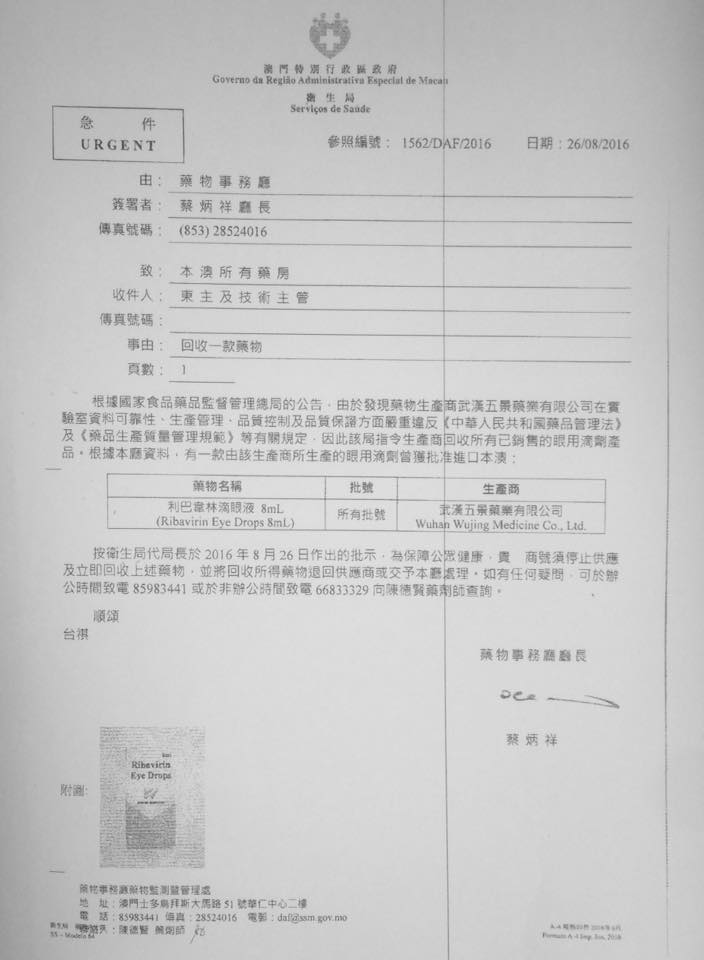
Pharmaceutical Affairs notices-drug recovery
Due to the producers location of the drug Monitoring Department ordered the producers to recover all production of ophthalmic preparations
Ribavirin eye drops 8ml
Ribavirin Eye Drop 8ml
The US FDA announced drug recovery
American drug recovery matters:
By the USA FDA in 2016 2 December approved the listing of the Cetylev effervescent tablets need to be recovered.
Since the drug packaging is not completely of reason and recycle.
Recovery batch number as follows:
Lot # 005C16, Exp date 2/2018
Lot # 006C16, Exp date 2/2018
Lot # 007C16, Exp date 2/2018
Cetylev(Acetylcysteine) 500mg effervescent tablets the main approved indications for the prevention and reduction of drug Paracetamol (acetaminophen)overdose and cause potential liver damage(hepatotoxic quantity of acetaminophen,APAP)
Acetylcysteine main role is to maintain and restore glutathione(Glutathione)levels, thereby reducing Paracetamol (acetaminophen)is the active metabolite of toxicity.