Category Archives: Notice
Pharmaceutical Affairs announcement-Drug Safety Information
Pharmaceutical Affairs announcement-Drug Safety Information
Due to Information drug interactions the recent increase in risk-tips for the pharmacy technician note
Pharmaceutical Affairs notices-doping medicine ingredients
Due to Hong Kong of the Drug Monitoring Department issued a notice,”Allmax Rapidcuts Shredded” within Western medicine ingredient-Yohimbine(Alpha-adrenoreceptor blocker).
But the products and no imports in Macau in batch, such as the citizens there are taking this product, immediately stop taking it, and to pharmaceutical professionals in the query.
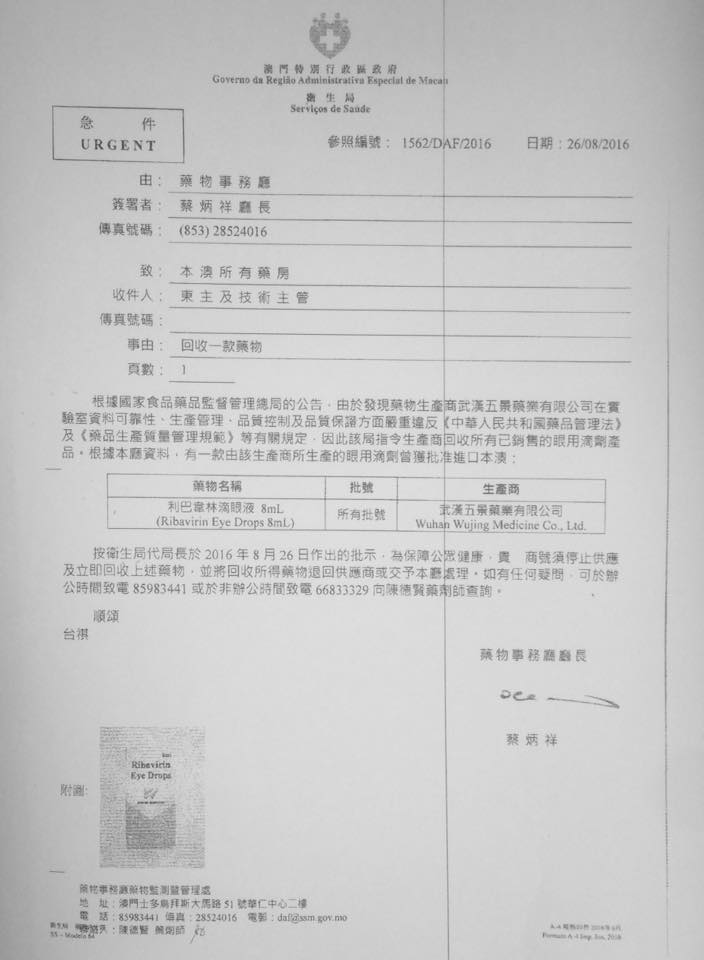
Pharmaceutical Affairs notices-drug recovery
Due to the producers location of the Drug Monitoring Department ordered the producers to recover all production of ophthalmic preparations
Ribavirin eye drops 8ml
Ribavirin Eye Drop 8ml
The US FDA announced drug recovery
American drug recovery matters:
By the USA FDA in 2016 2 December approved the listing of the Cetylev effervescent tablets need to be recovered.
Since the drug packaging is not completely of reason and recycle.
Recovery batch number as follows:
Lot # 005C16, Exp date 2/2018
Lot # 006C16, Exp date 2/2018
Lot # 007C16, Exp date 2/2018
Cetylev(Acetylcysteine) 500mg effervescent tablets the main approved indications for the prevention and reduction of drug Paracetamol (acetaminophen)overdose and cause potential liver damage(hepatotoxic quantity of acetaminophen,APAP)
Acetylcysteine main role is to maintain and restore glutathione(Glutathione)levels, thereby reducing Paracetamol (acetaminophen)is the active metabolite of toxicity.
Pharmaceutical Affairs notices-drug recovery
Pharmaceutical Affairs announcement
Due to Taiwan Food and Drug Administration issued notice,
Producers voluntarily recycle the following products
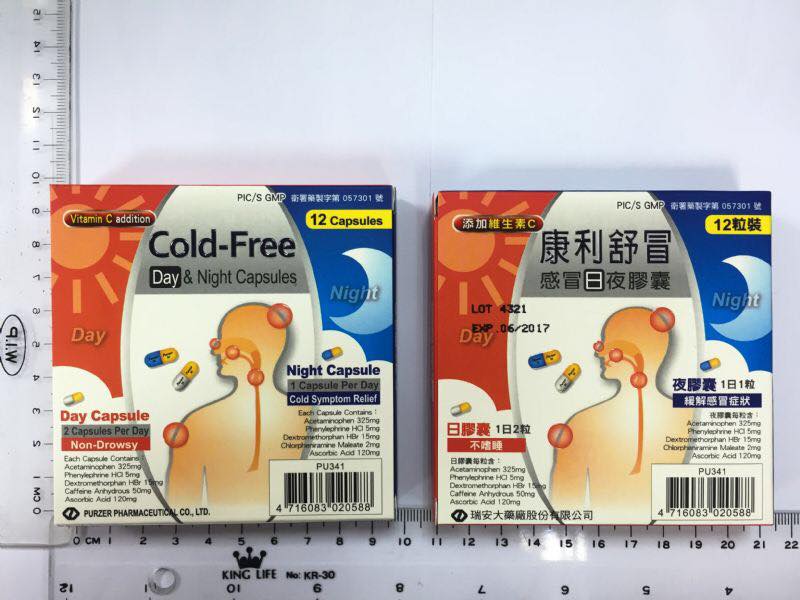
Conley Shu take flu day and night capsules
Cold Free Day n Night Capsules
Manufacturer: Taiwan Ryan large pharmaceutical factory Co., Ltd.
Batch: 4321
Pharmaceutical Affairs notices-drug recovery
Pharmaceutical Affairs announcement
Yongkang pharmaceutical entrance of the merchants-voluntarily recycle the following products
The Major Poly-Vita Drops 50ml
Batch: 20201601
20201603