Category Archives: Share
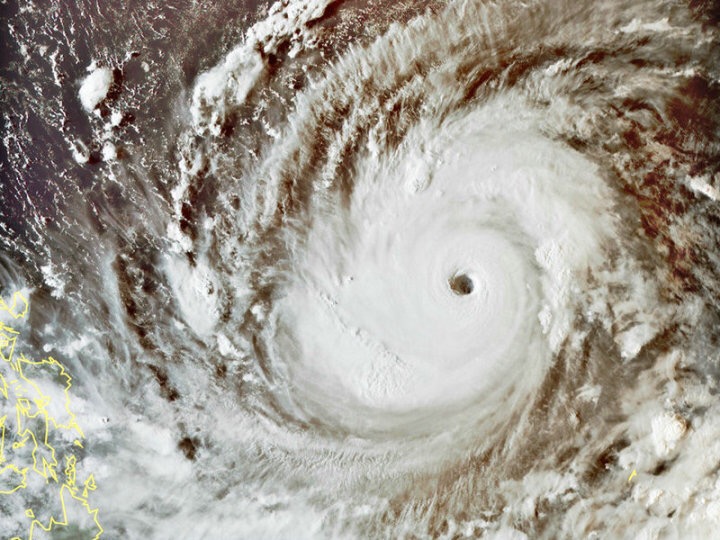
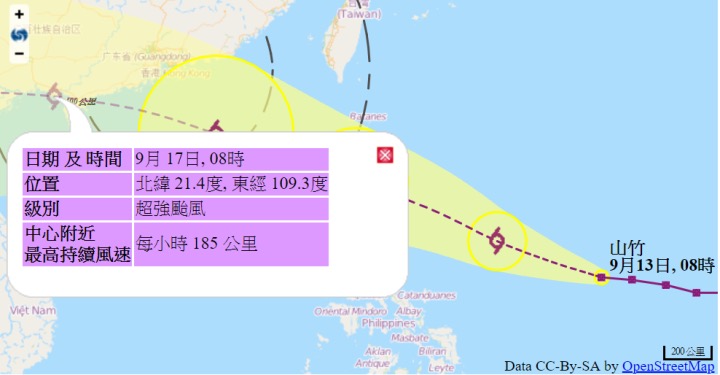
This year's Typhoon"mangosteen"prevention tips
The present O experienced in the previous year Typhoon this year after the current super Typhoon according the Hong Kong Observatory's latest forecast on Macau's influence still exists, although on a days pigeon Typhoon for Macao severely affected, and many pharmaceutical firms and pharmacy personnel to bring the inventory of the drug status of the work and clean shop and work environment.
On the figure for Hong Kong of"mangosteen"route prediction, substantially in the Macau Outer 150km after
Although the latest forecast of the"mangosteen"on the Macau influence is not"day of the Dove"route close to the Australian, but the trade name and the pharmacy personnel should be pre-placed on the ground or near the ground of the drug to move away from, to reduce the burst to a storm surge of drug damage and reduce economic loss, drug professionals should be to the residents to remind the pre-note themselves taking the drug the amount of is sufficient, such as inadequate should be in before the weekend to buy the right amount, in order to avoid suspension during the Typhoon the lack of drug so stop the drug, but also to remind the Don't panic store a large number of drugs in the home, in order to avoid drug store more than valid.
The representatives of Macau medical total 2018 cross-Strait conference
Every year, the Macau medical community Federation of organized cross-Strait meetings and add four to the medical speaker to present O medical personnel about new research findings and the latest medical development.
2018 Annual Meeting of the conference of the present will continue to dispatch representatives to participate, learn cross-Strait latest research results, common as Macau agent community advancement efforts. At the same time, this year's conference Chinese medicine services World Federation Youth Committee was formally established.
FDA approved Andexxa as anti-inhibition of Factor Xa class of drugs introduced to the market
The United States Food and Drug Administration(FDA)in today's time, announced approval of the first kind for anti-viral agents Andexxa market, for the treatment due to the use of a variety of inhibiting Factor Xa drugs(Rivaroxaban Xarelto ,and Apixaban on coagulation,Dabigatran up to a hundred students) is a life-threatening situation or cannot control bleeding, the FDA approved Portola Pharmaceuticals pharmaceuticals products in the United States market for sale.
Andexxa anti-viral agents is a synthetic protein fragment, its role and types of inhibition of Factor Xa drugs in combination, so that the body is full Factor Xa from inhibition and exerts its biological characteristics, and this new drug is mainly for is Rivaroxaban and Apixaban because they are currently also no formal antidote, while Dabigatran has its own pharmaceutical factory in the development of idarucizumab as an antidote, but its source is a monoclonal antibody, so according to the immunological idarucizumab organic will cause body hypersensitivity.
Andexxa in getting FDA approval to market will be in 2019 to 2023 market tracking survey(4 clinical), and the FDA also in this pharmaceutical on the box-shaped label because of its possible side effects include thromboembolism, ischemic risk of cardiac arrest and sudden death. And Andexxa in clinical practice when there is 5%of the patients with urinary tract infections and the Pneumonia of adverse reactions.
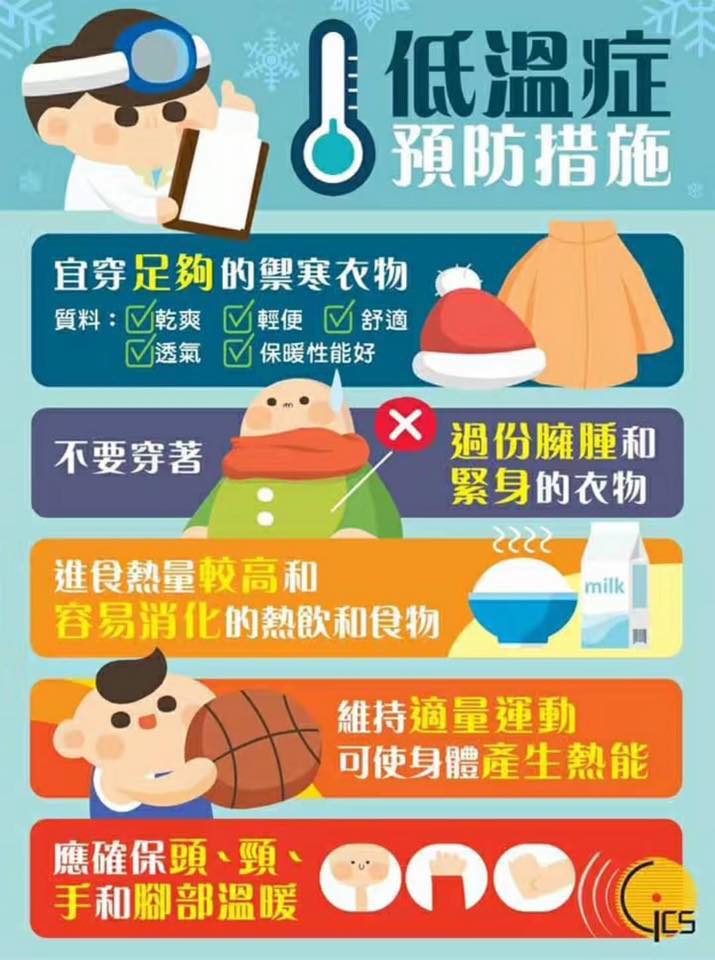
Please look out for hypothermia
Stepping into the winter, the Macao Meteorological Bureau forecast in this year's Christmas Eve and there will be a surge of cold air arrives in Macau, so everyone peers at work to keep an eye on the elderly may occur hypothermia symptoms. And this will be in advance good luck to each Trade Christmas and Happy New Year, the next year the Association will continue for everyone service.
The Macau government announced the Medical Malpractice Act and other administrative regulations
The government of the latest issue of the Gazette has announced the Medical Malpractice Act, related administrative content, the more there is
No. 3/2017 number of administrative regulations: the medical malpractice test to the Committee.
4/2017 number of administrative regulations: the medical dispute mediation center.
5/2017 number of administrative regulations: the health care provider professional liability compulsory insurance.
No. 45/2017 Executive Order No.: the approval of the health care provider professional liability compulsory insurance premiums and conditions.
No. 46/2017 Executive Order No.: approval of healthcare providers professional liability compulsory insurance unified policy design.
27th/2017 number of chief Executive approval: approval to provide medical records copy fee cap table.
28/2017 number of chief Executive instructions: the finalization of the application test fee.
《Marijuana is not a drug series
In fact, cannabis in medicinal properties on other than a restricted Poison be much higher.
Therefore, the United States, some States are classified as drugs control, but not every pharmacy can be supplied to the patient, so patients need only to specially provide cannabis formulation pharmacy Purchase.
Relive the knowledge:
Parkinson's disease(Parkinson’s disease (PD) patients, the largest feature occurs when the EPS(Extrapyramidal Syndromes, or called extrapyramidal symptoms, which factor is the brain neurotransmitter of the proportion of abnormalities in 5-HT increase or Dopamine to reduce in order to make the brain 5-HT and Dopamine relative proportions of change and emergence, but to note the emergence of EPS does not necessarily have Parkinson's.
While cannabis is the main ingredient THC(THC), you can make the neurons to secrete more Dopamine so that the brain transfer of substances the ratio of re-to give balance and order to the EPS symptoms reduced.
Interesting drug share
Pills dissolve believe everyone in the University curriculum experiment has ever seen.
But there is a part of the drug factory for the production of pills or the exterior of the capsule is not dissolved in the body and the discharge, by itself the agent design is to make the users taking daily just a grain of it is sufficient or have other special considerations. So usually labeled as XL, EL, EX, LA, CR, etc., because it is the pharmaceutical factory registration name is different, this is because the commercial marketing considerations, but refers to long-term(Prolonged-release,Extended release etc.)
But not all long-acting(Prolonged-release)drug the outside will not dissolve, mainly the drug companies drug design.
Common:Adalat OROS 30mg(otherwise 60mg, but note that Adalat 5mg does not belong to the long-term, and the drug factory has been discontinued 5mg)