The Macau health Bureau announced the continuing professional development of the test plan
Macau health Bureau in 2015 to the health professionals explain medical professionals when presented with each of the registered professionals required for continuing professional development plan(CPD plan), and in the today's Department of health to registered the medical professionals sent letters, informing the CPD plan was officially launched.
This plan is experimental, so the current year(by 2018/7-month-2019/6 month)between the CPD requirements and does not affect the renewal of the license, professionals may voluntarily put eligible CPD supporting documents to the Department of Health license section or the professional belongs to the licensing Agency. This year the test may also be so that the agent is the professional community for the new health care professional act among the CPD requirements are more clearly aware of the process and prepare for the transition to the new Act came into force among the mandatory in-service CPD programme.
The current CPD plan for substantially 1 hour to 1 credit, but because CPD credits included in the project vary, mainly according to the health Bureau files in the content calculation. Each professional per year of CPD credits are different to the pharmacy Professional, the pharmacist is temporarily the annual need 25 credits, and a pharmacy technician for 20 credits.
Response pharmacy professional higher number and for the local agent personnel professional promotion is the one of the purposes of the future this will also be the opening of more events and conferences, to allow more experts to share their research and clinical treatment experience. That the Macao public to have better drug safety and with the latest scientific research standards.
For CPD details can beClick on the CPD plan page

FDA approved Andexxa as anti-inhibition of Factor Xa class of drugs introduced to the market
The United States food and Drug Administration(FDA)in today's time, announced approval of the first kind for anti-viral agents Andexxa market, for the treatment due to the use of a variety of inhibiting Factor Xa drugs(Rivaroxaban xarelto ,and Apixaban on coagulation,Dabigatran up to a hundred students) is a life-threatening situation or cannot control bleeding, the FDA approved Portola Pharmaceuticals pharmaceuticals products in the United States market for sale.
Andexxa anti-viral agents is a synthetic protein fragment, its role and types of inhibition of Factor Xa drugs in combination, so that the body is full Factor Xa from inhibition and exerts its biological characteristics, and this new drug is mainly for is Rivaroxaban and Apixaban because they are currently also no formal antidote, while Dabigatran has its own pharmaceutical factory in the development of idarucizumab as an antidote, but its source is a monoclonal antibody, so according to the immunological idarucizumab organic will cause body hypersensitivity.
Andexxa in getting FDA approval to market will be in 2019 to 2023 market tracking survey(4 clinical), and the FDA also in this pharmaceutical on the box-shaped label because of its possible side effects include thromboembolism, ischemic risk of cardiac arrest and sudden death. And Andexxa in clinical practice when there is 5%of the patients with urinary tract infections and the pneumonia of adverse reactions.
The present will participate in the 2018 Macau Obstetrics and Gynecology medicine exhibition
In this Sunday, Macau, Obstetrics and Gynecology physicians Association annual exhibition, with more than 20 pharmaceutical companies participated in the exhibition and to the health care professional to explain the latest products and associations also add to the digital senior doctors participated in the seminar,which explains endometriosis medication, references in the literature to explain the pure progesterone than oral contraceptives used to control the disease with relatively good results.

The US FDA announced the recovery Daclizumab (Zinbryta)
The United States drug and food Administration(FDA)in the recently announced full recovery of the United States all of Daclizumab formulation, recovery is the FDA to enforce and assist the drug companies(Biogen And AbbVie)to recall all containing Daclizumab medicines.
Daclizumab trade name Zinbryta is a treatment for relapsing multiple sclerosis Multiple Sclerosis, And Daclizumab is a novel fully human source of anti-Tac monoclonal antibody, and its role in IL-2 receptacle α chain. And in this month 2, There are reports of this drug affects the liver and immune problems. In Europe appeared a total of eight cases of severe side effects, and administration of the factory statement that Daclizumab will be in the global recovery and provide only Daclizumab to 2018 4 June 30 to have the clinical needs of the institution.
And in the Macau market, containing Daclizumab formulation is part of hospital medicine(UH), and the inlet makes for Roche, on the Macau market recovery or not invited to the Macau health Bureau query, while the patient to your attending doctor queries.

The Lantern Festival happy
I wish all the guilds the Lantern Festival happy,healthy. Spring more to watch out for flu transmission

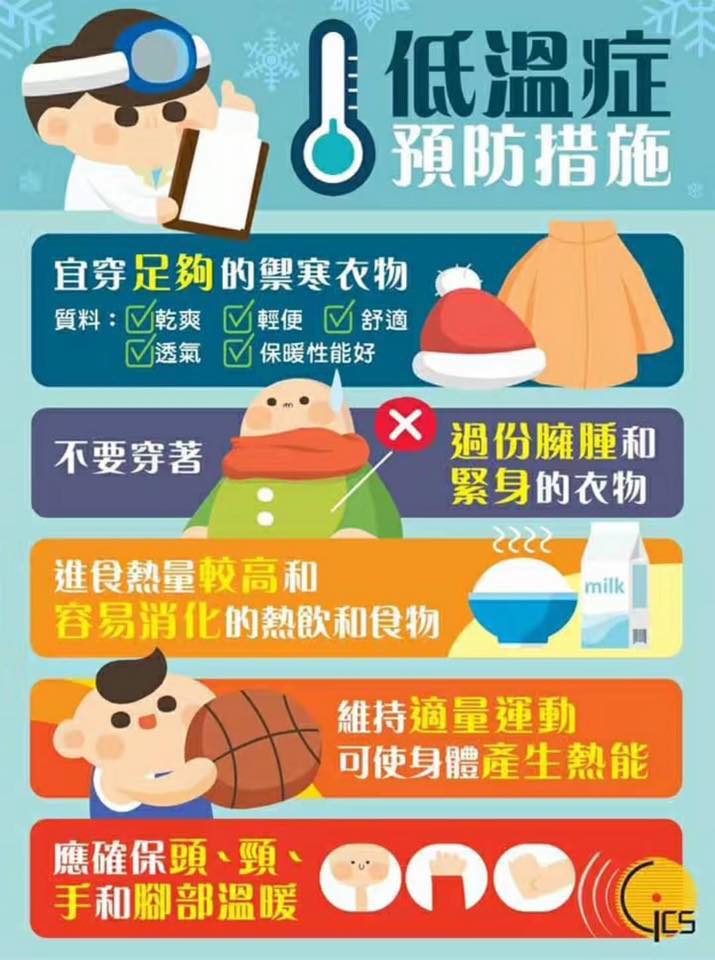
Please look out for hypothermia
Stepping into the winter, the Macao meteorological Bureau forecast in this year's Christmas Eve and there will be a surge of cold air arrives in Macau, so everyone peers at work to keep an eye on the elderly may occur hypothermia symptoms. And this will be in advance good luck to each trade Christmas and Happy New Year, the next year the Association will continue for everyone service.
After a Typhoon the drug Safety matters
This will inform the industry of:
Bear in mind that the moisture of the drugs are not suitable for use, even if it has not expired of the same drug need to destroy.
While the need for refrigeration of the drug because it is the Typhoon caused large power outages may make the drug not in the best state, so also should the refrigeration of the drugs sent for destruction, and to the patient medication safety as the first consideration.
In addition, each of the peers remember as a resident for refrigerated medications and other issues, to review drug descriptions and answer, especially insulin SC injection of the drug, because the type is various, the answer to the patient when more need to be careful.
Due to the Typhoon power strong, so that the Macao throughout the risk, Interbank commute more careful road condition, in order to avoid injury.
The new hilltop hospital prescription drug supply agreement briefing
On Friday, hilltop hospital for each Protocol pharmacy Conference, of which new provisions will be in October this year, when pursued
The new hilltop hospital prescription drug supply agreement instructions will focus on the following:
1. Protocol pharmacy want to install new networking lines and plans.
2. The network requirements of the application fixed the URL(IP address)
3. Require a computer to the Department of health to install the application software.
4. Application by the health authority personnel to install.
5. The software is responsible for recording the OPD of the drug entry and the dispatch number and sent the number of registration is to scan the OPD on the prescription bar code.
6. As the pharmacy in handing OPD drug on a single drug, found the number insufficient and not distributed the remaining amount within seven days.
7. Protocol pharmacy during opening hours must have a supervisor or technician when the value of.
8. Evening-in-Office of Protocol pharmacy need two employees of OPD prescriptions be formulated and determined.
9. Agreement of the pharmacy may not order any form of discount to attract patients.
10. The above content by the finishing, but in the end is the peak hospital interpretation shall prevail.
The Macau government announced the medical malpractice Act and other administrative regulations
The Government of the latest issue of the Gazette has announced the medical malpractice act, related administrative content, the more there is
No. 3/2017 number of administrative regulations: the medical malpractice test to the Committee.
4/2017 number of administrative regulations: the medical dispute Mediation Center.
5/2017 number of administrative regulations: the health care provider professional liability compulsory insurance.
No. 45/2017 Executive Order No.: the approval of the health care provider professional liability compulsory insurance premiums and conditions.
No. 46/2017 Executive Order No.: approval of healthcare providers professional liability compulsory insurance Unified policy design.
27th/2017 number of Chief Executive approval: approval to provide medical records copy fee cap table.
28/2017 number of Chief Executive instructions: the finalization of the application test fee.
Medical personnel insurance purchase description
Statement
The present learn the collection of premiums and the application form will be forwarded to ACE insurance company, and in the evening on 23 May evening about the purchase of insurance will, due to the seats not necessarily enough, members of the industry to the scene after as a result of the seat shortage may coordinate a person questions and listen to notes and then forwarding, and insurance, learn just is responsible for the collection of premiums and the application form, relating to the insurance content and follow-up details or claims should directly ask the insured with ACE Insurance Co., Ltd., Tel:2855 7191 to.